UDC 635.34:632.3
A.N. Ignatov, S.V. Panchuk, Vo Thi Ngok Ha, E.S. Mazurin, K.A. Kromina,
F.S. Dzhalilov
Summary. It was an evidence of wide-spread epidemics of black rot on cabbages in Russia at 2015. The studied genetic parameters indicates higher genetic uniformity and virulence of new strains. The resistance genes in cabbage cultivars and other brassicas were identified based on gene-for-gene interaction with different races of the pathogen. Some cabbage cultivars seemed to carry the homologous genes for race-specific resistance. It is suggested that non-specific stem resistance found in Chinese kale, broccoli and cabbage might be an alternative means of genetic protection against the pathogen.
Keywords: Xanthomonas campestris pv. campestris, gene-for-gene interaction, races, seed infection.
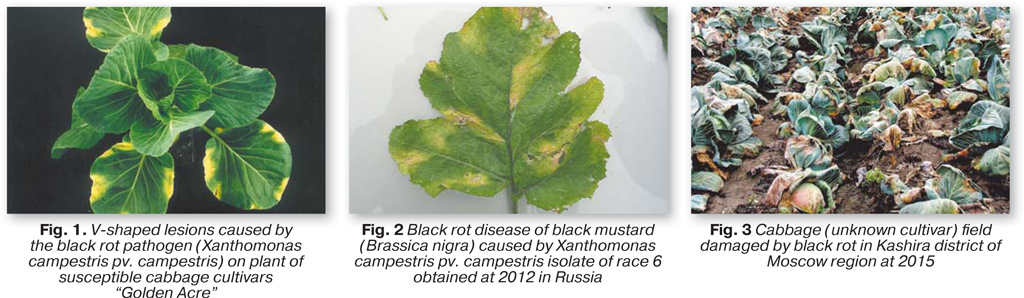
Incidence of black rot caused by Xanthomonas campestris pv. campestris (Xcc) on horticultural brassicas is well recognized worldwide. Periodical epidemics of the disease were usually ascribed to the introduction of susceptible cultivars, careless application of contaminated seeds and seedlings and weather conditions favorable for disease development [1]. The first description of the disease was made by Garman in 1894 in Kentucky, USA. Black rot can attack all cultivated brassicas and it is probably the most important disease of Brassica oleracea L. crops in the world. Black rot is a seed-borne, vascular disease; the bacteria penetrate the plant through the hydathodes or wounds. The main symptoms are V-shape chlorotic, yellow lesions at the margins of the leaves, with darkening of veins; the affected leaves can drop prematurely and distortion of leaves, dwarfing and plant death can also occur.
Use of healthy planting material (seeds and transplants) and agotechnology that limit the spread of the disease are principal ways to control the disease in field. The development and use of resistant cultivars has had a limited success in practice due to the small number of useful sources of resistance available and race-specific reaction of the known types of resistance.
Identification and origin of Xcc races
It was shown that Xcc is composed of genetic and serologically heterogeneous groups of strains. Isolates of Xcc can be separated into nine different races on the basis of the reaction of cultivars of B. oleracea, B. rapa, B. napus, B. carinata and B. juncea [2]. Races 1, 3, and 4 are predominant in B.oleracea crops. Evidence of new races was suggested on the base of interaction between worldwide collection of Xcc and new differential varieties in B. oleracea and B. napus. Comparison of the pathogen strains collected at 2012 in different regions of Russia and other former USSR countries to the previously studied population by race typing, MLST, and rep-PCR showed considerable genetic change occurred between 2007 and 2012, associated with increasing incidence of the black rot disease. The studied genetic parameters indicates higher genetic uniformity and virulence of new strain group, which can overcome most of resistance types in current commercial cabbage cultivars. Only three F1 hybrids: «Sintex», «Braxan», and «Cerox» showed significant tolerance to strains of this new population.
Sources and inheritance of resistance
Most B. oleracea plants are susceptible to all races of the pathogen, some accessions had resistance to one or more of the rare races (2, 3, 5 and 6). Strong resistance to race 4 is frequent in B. rapa and B. napus. Resistance to races 1 and 4 was present in some B. nigra and B. juncea accessions. Potential broad spectrum resistance was found at a low frequency in B. rapa, B. nigra and B. carinata [3, 4]. The inheritance of resistance to three races of Xcc was studied in B.oleracea, B. carinata and B. napus [4].
Studies on the recent outbreaks caused by X. campestris on cabbage in Russia suggested that spreading of new highly aggressive variants of the pathogen was the main reason for these epidemics [5]. However, breeding of B. oleracea for resistance to black rot has been undertaken without recognition of the existence of pathogenic variants (races). As a result, control of the disease by the introduction of some resistant cultivars may not be effective.
The variability of Xcc continuously endangers cultivars with a narrow genetic base of resistance. The spreading of the disease on new host crops considerably increases the chance of outbreaks on more susceptible vegetables grown in the same locations. Pathotyping of the pathogen populations may be necessary to provide a scientific basis for breeding and introduction of resistant varieties in the areas endangered by black rot.
Since the pathogen can remain in soil even in plant debris only for 1 or 2 growing seasons [6], survival in contaminated seeds is considered to be most essential for the cycle of the disease.
Ability of the pathogen to multiply in the vascular system of plant plays a major role in the expression of black rot symptoms. Vein plugging in plants infected with Xcc seems to be due to the accumulation of fibrillar material in vessels to prevent pathogen spreading inside the vascular system.
The resistant reaction in plant occurs in hydathodes, the natural gateway for the pathogen penetration into plant, and in the vascular system, where the pathogen spreads and multiplies. In early studies, a difference between leaf and stem susceptibility of cabbages was noticed. With the same leaf reaction as in European cultivars, the Japanese cultivars exhibited a lower stem susceptibility. This novel stem resistance can be represented as the arrest of the pathogen in the stem vascular system. It was observed that in the progeny of a cross between stem-resistant Chinese kale and leaf-resistant cabbage, these types of plant reaction were controlled by different genes and could be evaluated separately [7]. After these recent achievements, sources of race-specific leaf and non-specific stem resistance among B. oleracea became available for plant breeding. The discovery of the race structure of Xcc populations for the first time enabled to design a breeding program based on the recognition of different genes and different mechanisms of resistance to black rot.
An integrated, comprehensive program is needed to manage black rot successfully:
- Resistant varieties will improve performans of bactericide applications before development of symptoms. Resistant varieties have fewer infection sites and/or the affected area is much smaller compared with susceptible varieties.
- Minimize chance of seed or transplants being infested: use seed or transplants certified to be free of Xcc; use seed treated by hot water or dry heat. For hot water treatment use 48-50°C water for 20-30 minutes.
- If seedlings are grown in a greenhouse, use new or sterilized flats and soilless mix.
- Locate seedbeds away from production fields in an area where crucifers have not been grown for at least 2 years.
- Inspect seedlings routinely. If symptoms are found early, destroy seedlings in that area.
- Foliage should be dry when seedlings are transplanted.
- If possible, use direct seed production fields because bacteria can spread much more extensively among plants in seedbeds.
- Work in fields only when foliage is dry, especially if black rot is present.
- Control insects and cruciferous weeds.
References
- Williams, P.H. Black rot: a continuing threat to world crucifers, Plant Disease, 1980, Vol. 64, pp. 736-742.
- Vicente, Joana G., Conway, J., Roberts, S. J. and Taylor, J. D. Identification and origin of Xanthomonas campestrispv.campestris races and related pathovars, Phytopathology, 2001, Vol. 91, No 5, pp. 492-499.
- Vicente, Joana G., Taylor, J. D., Sharpe, A. G., Parkin, I. A. P., Lydiate, D. J. and King, G. J.. Inheritance of race-specific resistance to Xanthomonas campestris pv. campestris in Brassica genomes, Phytopathology, 2002, Vol. 92, No. 10, pp. 1134-1141.
- Ignatov, A., Kuginuki, Y. and Hida, K. Black rot of crucifers and sources of resistance in brassicas, Japanese Agricultural Research Quarterly, 1998, Vol. 32, pp. 167-172.
- Vo Tkhi Ngok Kha, F.S. Dzhalilov, E.S. Mazurin, E.I. Kyrova, S.V. Vinogradova, N.V. Shaad, D. Laster, A.N. Ignatov. Rasprostranenie novogo genotipa Xanthomonas campestris pv. campestris v Rossii v 2012 g. (Spread of new genotype of Xanthomonas campestris pv.campestris in Russia in 2012), Zashchita kartofelya, 2014, No2. pp. 28-30.
- Schaad, N.W. & White, W.G. Survival of Xanthomonas campestris in soil, Phytopathology, 1974, Vol. 64, pp. 1518-1520.
- Ignatov, A., Kuginuki, Y., and Hida, K. Vascular stem resistance to black rot in Brassica oleracea, Canadian journal of botany, 1999, Vol. 77, No. 3, pp. 442-446.
About authors
A.N. Ignatov, DSc,
professor of Russian University of People’s Friendship, Research Director of OOO Research Center “Phytoengeneering”. E-mail: an.ignatov@gmail.com.
S.V. Panchuk, postgraduate student, All-Russian Research Institute of Vegetables Breeding and Seed Production.
E-mail: s.v.panchuk@mail.ru,
Vo Thi Ngoc Ha, OOO Research Center “Phytoengeneering”,
E-mail: ngochavo.88@gmail.com.
Mazurin E.S., PhD, Vice-director, All-Russian Research Institute of Agricultural Biotechnology.
E-mail: zarauh@mail.ru.
Kromina K.A., PhD, senior researcher, Institute of General Genetics by N.I. Vavilov.
E-mail: krominaks@yahoo.com
Dzhalilov F.S., DSc., Professor, Russian State Agrarian University – MSKHA by K.A. Timiryazev,
E-mail: labzara@mail.ru.
Сосудистый бактериоз крестоцветных в России – причины эпифитотии, методы защиты и источники селекции на устойчивость к болезни
Игнатов Александр Николаевич, доктор биол. наук, профессор ФГАОУ ВО РУДН, зам. директора ООО ИЦ «Фитоинженерия». E-mail: an.ignatov@gmail.com.
Панчук Сергей Владимирович, аспирант ФГБНУ ВНИИССОК
E-mail: s.v.panchuk@mail.ru.
Во Тхи Нгок Ха, канд. биол. наук, сотрудник ООО ИЦ «Фитоинженерия»,
E-mail: ngochavo.88@gmail.com.
Мазурин Евгений Сергеевич, канд. биол. наук, зам. директора ФГБНУ ВНИИСХБ, E-mail: zarauh@mail.ru.
Кромина Ксения Андреевна, канд. биол. наук, с. н. с. ФГБНУ ИОГЕН имени Н. И. Вавилова РАН,
E-mail: krominaks@yahoo.com.
Джалилов Февзи Сеид-Умерович, доктор биол. наук, профессор, зав. лаб. ФГБОУ ВО «Российский государственный аграрный университет – МСХА имени К.А.Тимирязева» .E-mail: labzara@mail.ru.
В 2015 году в России наблюдалась эпифитотия сосудистого бактериоза. Результаты изучения генетических параметров указывают на более высокую генетическую однородность и вирулентность новых штаммов. Гены устойчивости в сортах капусты и других крестоцветных были определены на основе межгенного взаимодействия с различными расами патогена. Некоторые сорта капусты, возможно, несут гомологичные гены расово-специфической устойчивости. Предполагается, что неспецифическая вертикальная устойчивость, обнаруженная у китайской капусты, брокколи и белокочанной капусты может быть альтернативным средством геетической защиты от патогена.
Ключевые слова: Xanthomonas campestris pv. campestris, межгенное взаимодействие, расы, семенная инфекция.